Case of the Week #626
(1) Slovakia; (2) Kasr Al Ainy teaching hospital, Egypt; (3) UCSF Health, San Francisco, California, USA
A woman with no significant medical history presented to our center at 20 weeks and 5 days for fetal anatomic survey. The following findings were observed:
View the Answer Hide the Answer
Answer
We present a case of Arthrogryposis multiplex congenita with Pena-Shokeir phenotype. Microarray analysis revealed a 240kb duplication of chromosomal region 6q23.3 which includes the genes AHII1 and LINC00271. The Abelson Helper Integration Site 1 (AHI1) gene is associated with Joubert syndrome which is characterized by hypotonia, nystagmus, abnormal respiratory activity, and central nervous system anomalies such as hypoplasia of the cerebellar vermis, polymicrogyria, and corpus callosum malformations. Molecular genetic testing using the Multiplex Ligation-Dependent Probe Amplification (MLPA) method did not demonstrate any mutations in SMN genes, which are related to spinal muscular atrophy. The patient opted for termination of pregnancy.
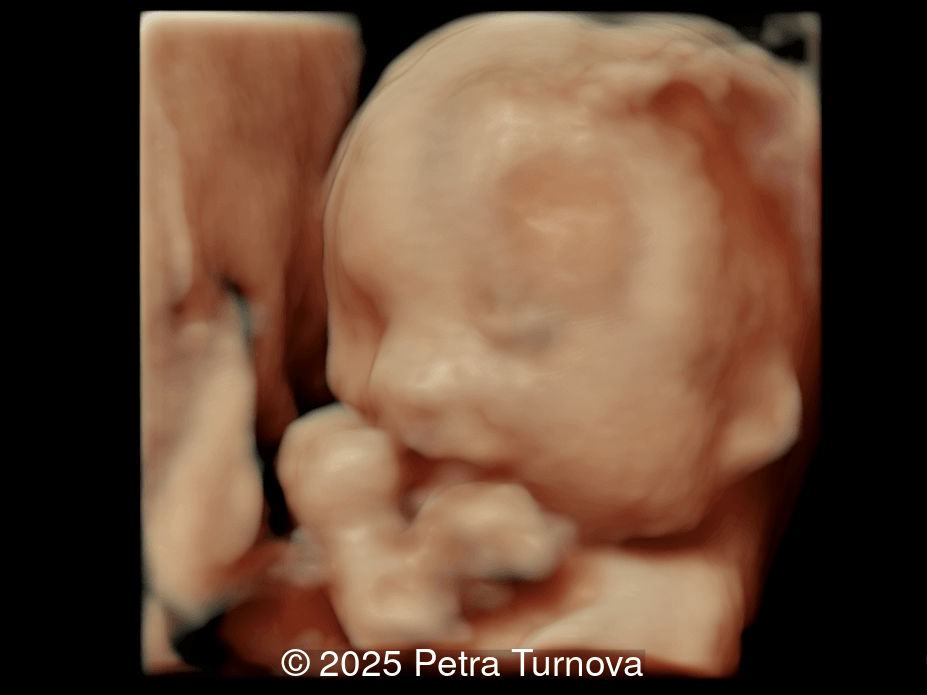
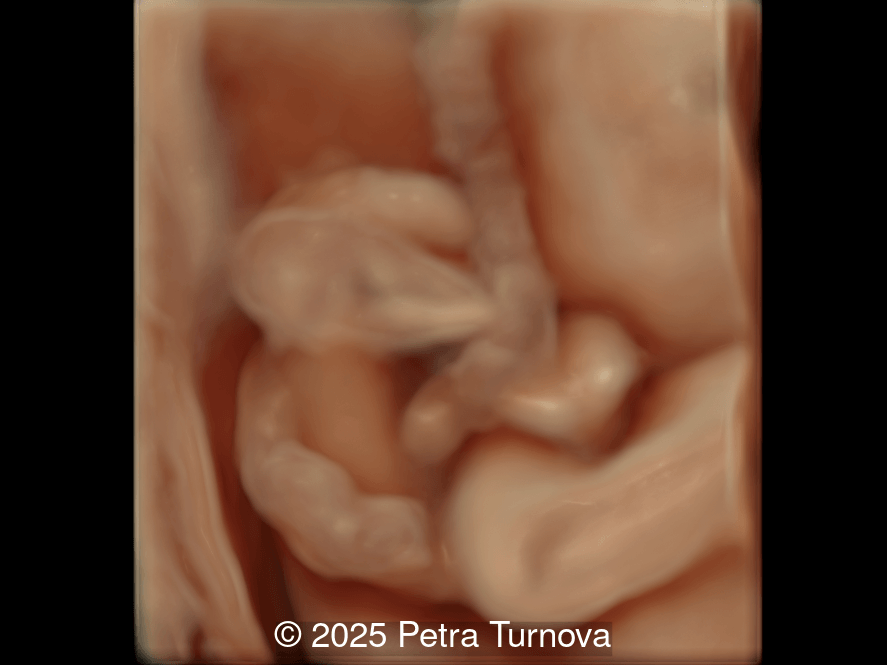
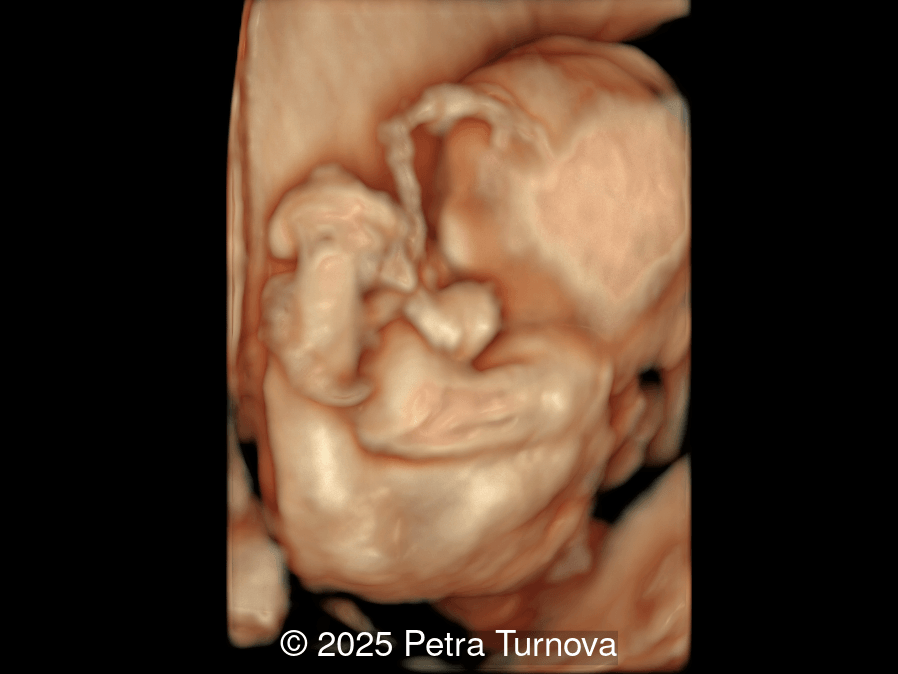
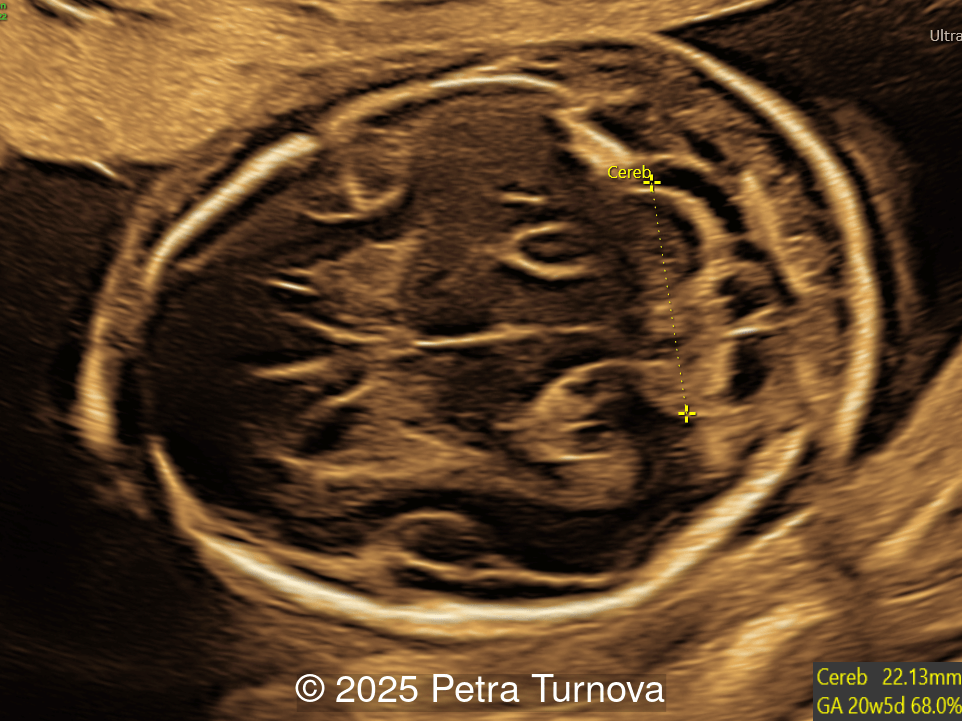
Our imaging revealed malformations in all four limbs with elbow, wrist, and knee joints fixed in extension, as well as foot and ankle malformations. Profile abnormalities were observed with prefrontal edema, flat profile and retro-micrognathia. Ultrasound demonstrated minimal stomach filling related to a lack of swallowing ability and hypoplastic lungs due to poor diaphragmatic movement. Nuchal edema was present.
Discussion
Arthrogryposis multiplex congenita (AMC) describes a phenotype that can be caused by many disease processes, and is characterized by multiple contractures affecting at least two different body areas. The term “arthrogryposis” is derived from Greek meaning “curved and hooked joints” [1,2]. The prevalence for multiple congenital contractures is approximately 1 in 4300 to 5100 cases [3,4]. Fetal joints begin developing in the 5th and 6th weeks of gestation, and adequate fetal movement is essential for their proper formation. Lack of movement causes connective tissue to accumulate in the joints, leading them to become stiff and contract. This results in hypoplasia of the muscles in the affected limbs, which are replaced partially or completely by fat and fibrous tissue [1,5]. Arthrogryposis multiplex congenita thus develops due to fetal akinesia during these critical weeks of limb development and usually involves the extremities but may also involve the jaw, neck, and spine [6].
Arthrogryposis is only diagnosed prenatally in 26-37% of pregnancies [6,7], which is likely due to the time-consuming nature of fetal limb movement testing, the requirement for an experienced examiner, and the difficulty of diagnosis in the first trimester [5]. Studies have demonstrated that isolated movement of the fetal limbs can be seen sonographically as early as 8 weeks gestation [8] and abnormal fetal motor and postural findings can be detected as early as 11 weeks [9,10]. However, arthrogryposis is usually diagnosed in the second trimester [6,11]. In a survey study reviewing over 300 patients diagnosed with arthrogryposis multiplex congenita, the most common ultrasound findings in fetuses were clubfoot (83%), little or no fetal movement (53%), and clenched fists (51%). Joint contractures were noted in the elbows of 51% and knees of 46% of fetuses [6]. Therefore, if there is maternal concern for lack of fetal movement, presence of clubfoot or clenched fist, the diagnosis of arthrogryposis should be excluded and an experienced ultrasound technician should evaluate fetal movement for up to 45 minutes. In a normal fetus before 20 weeks of gestation, the longest period of inactivity is 13 minutes, while fetuses after 30 weeks may be inactive for 45 minutes [12]. Indirect ultrasound signs of reduced fetal movement are polyhydramnios and collapsed stomach which may be due to impaired swallowing, and facial anomalies such as micro- or retrognathia. Disuse of limbs can lead to reduced bone growth, hypoechogenicity, hypomineralization and fractures of the long bones, as well as the persistence of breech position [13].
Since club foot is the most commonly identified contracture in arthrogryposis, this finding should prompt a thorough evaluation of other joints, with documentation of the number affected and the position in flexion, extension, or dislocation [13]. Organ systems should be assessed for additional abnormalities. In a retrospective study reviewing 41 pregnancies with arthrogryposis multiplex congenita, hydrops, nuchal edema, scoliosis, and absent stomach filling were significantly associated with fetal or neonatal demise [10,14]. The presence of lung hypoplasia, pterygia, polyhydramnios, growth restriction, central nervous system malformations including microcephaly and enlargement of the ventricles are usually indicative of a severe, often lethal condition [10,15]. Fetal MRI can be performed to identify abnormalities of the central nervous system, which may suggest a neurologic etiology of arthrogryposis. Additionally, fetal MRI can assess lung volumes, which may be underdeveloped as a result of hypotonia [1,16].
Disease processes that lead to abnormalities of the central nervous system, peripheral nerve development, muscle, connective tissue, and bone, as well as external compression can all cause arthrogryposis multiplex congenita (Table 1). Neuromuscular abnormalities are the most common cause of arthrogryposis and encompass approximately 75% of cases [4]. Chromosomal aneuploidy or genetic syndromes result in defects in neural migration leading to developmental brain abnormalities such as cerebral and cerebellar hypoplasia, holoprosencephaly, pyramidal tract degeneration, olivopontocerebellar degeneration, and cortical frontal atrophy. Chromosomal anomalies associated with arthrogryposis include trisomy 18, 13, 21, as well as translocations of chromosome 9q and microdeletions of 5q23 [17]. This is the first case report of Joubert syndrome associated with arthrogryposis multiplex congenita. Patients with brain abnormalities causing arthrogryposis often have severe learning disorders, epilepsy and developmental delay in addition to multiple joint contractures. In a study of 315 families with arthrogryposis, 11% of patients had intellectual disability and 6% had epilepsy [11].
This is the first case report of Joubert syndrome associated with arthrogryposis multiplex congenita. Joubert syndrome is characterized by hypotonia, abnormal ocular movement, hyperpnea, developmental delay, hypoplasia of the cerebellar vermis and brainstem malformation. The molar tooth sign seen on axial MRI of the brain is a characteristic finding in Joubert syndrome and demonstrated by triad of malformations including (1) a deepened interpeduncular fossa with narrow isthmus, (2) thickened, elongated and horizontally oriented superior cerebellar peduncles as a result of the absence of normal decussation and (3) a variable degree of vermian hypoplasia. Mutations in nine genes have been implicated in Joubert syndrome, including AHI1 [18].
Conditions affecting the spinal cord, the peripheral nervous system, and the muscles can present with arthrogryposis multiplex congenita. Spinal cord disorders include spinal muscular atrophy including Werdnig–Hoffmann disease. Amyoplasia is characterized by symmetric poor development of limb muscles, and is the most common form of arthrogryposis multiplex congenita occurring in approximately 20-25% of cases [2,4]. The condition is sporadic and thought to occur due to atrophy or dysgenesis of the affected limb’s anterior horn cells suggesting a vascular insult to the developing fetus between the 8th and 11th week of gestation [19]. Approximately 10% have gastroschisis or intestinal atresia [1,20]. Conditions affecting the peripheral nervous system include demyelinating disorders. Muscle disorders that present with arthrogryposis include congenital muscular dystrophy, myopathies, intrauterine myositis and mitochondrial disorders [1]. Congenital muscular dystrophies are the result of abnormal function of the dystrophin-glycoprotein–associated complex in the sarcolemma of skeletal muscle and include Ullrich syndrome, Walker–Warburg syndrome, Fukuyama, and merosin-deficient congenital muscular dystrophies [20]. Distal-type arthrogryposis is a collection of 12 distinct conditions often with autosomal dominant inheritance that share distal extremity contractures as a defining feature [1,5]. Many of the affected genes, such as TNNI2 (troponin 1), TNNT3 (troponin T), TPM2 (tropomyosin 2), MYH3 (myosin-3), and MYBPC1 (myosin binding protein C), encode for components of the fast-twitch muscle contractile apparatus primarily found in the hands and feet [21]. Multiple pterygium syndrome represents a group of disorders caused by a mutation in the acetylcholine receptor and can be broadly divided into lethal and nonlethal forms (Escobar syndrome). The lethal form is characterized by intrauterine growth restriction, multiple pterygia, hydrops, dysmorphic facies and lung hypoplasia. Nonlethal form (Escobar syndrome) is characterized by joint webbing, scoliosis, and dysmorphic facial features [1,19]. In conditions primarily affecting the peripheral nervous system and muscles, the patients tend to have normal intelligence.
Extrinsic factors that can affect the fetal neuromuscular system include teratogenic agents, intrauterine infections, and maternal conditions. Teratogens such as misoprostol, cocaine, and alcohol have been associated with arthrogryposis [1,22]. Cocaine can cause diffuse brainstem and spinal cord neuronal degeneration and focal cerebral infarction, consistent with acquired intrauterine ischemic damage [23]. Maternal infections can cause fetal akinesia and neurologic compromise and include cytomegalovirus, toxoplasmosis, varicella, rubella, and zika. Additionally, zika has been associated with microcephaly [17,20]. Maternal conditions include multiple sclerosis, myotonic dystrophy [13] and myasthenia gravis, which results in placental transfer of maternal acetylcholine receptor antibodies and transient neonatal myasthenia gravis [20].
Other conditions that cause arthrogryposis multiplex congenita affect the connective tissue, cartilage and bone and include systemic connective tissue diseases such as congenital contractural arachnodactyly, Larsen syndrome, Ehlers–Danlos syndrome, and skeletal dysplasias [1,20]. Patients with congenital contractural arachnodactyly, also known as Beals syndrome, have an autosomal dominant mutation in FBN2 (fibrillin-2) resulting in scoliosis, arachnodactyly and “crumpled ears”. Larsen syndrome is an autosomal dominant condition that results from a mutation in FLNB (filamin B) and most commonly presents with anterior dislocation of the knees [1,5,20]. Factors extrinsic to the fetus such as intrauterine compression resulting from multiple pregnancy, oligohydramnios, or uterine anomalies can also lead to arthrogryposis [1,20].
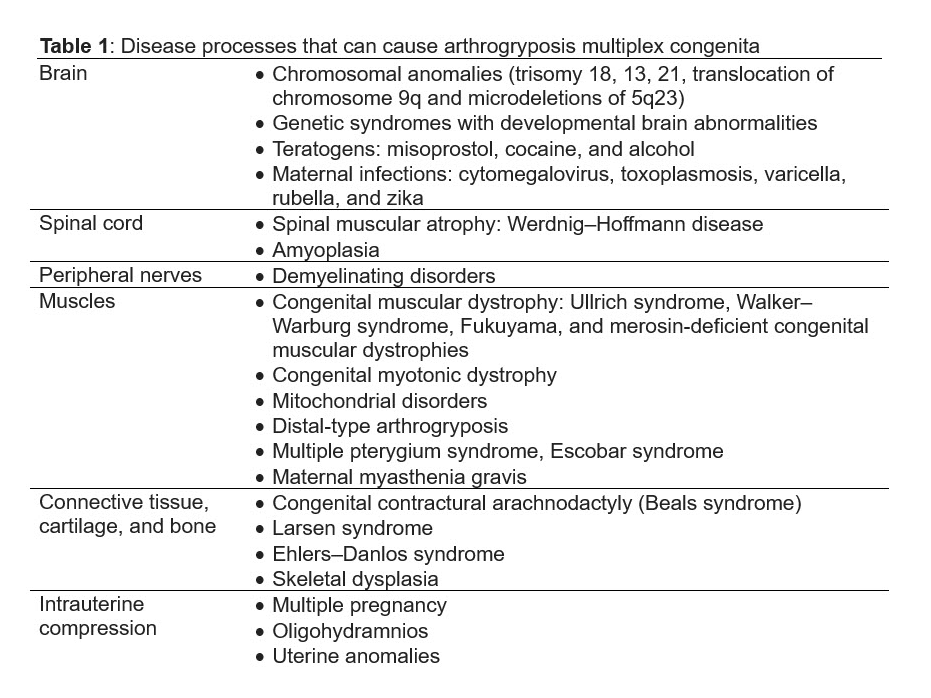
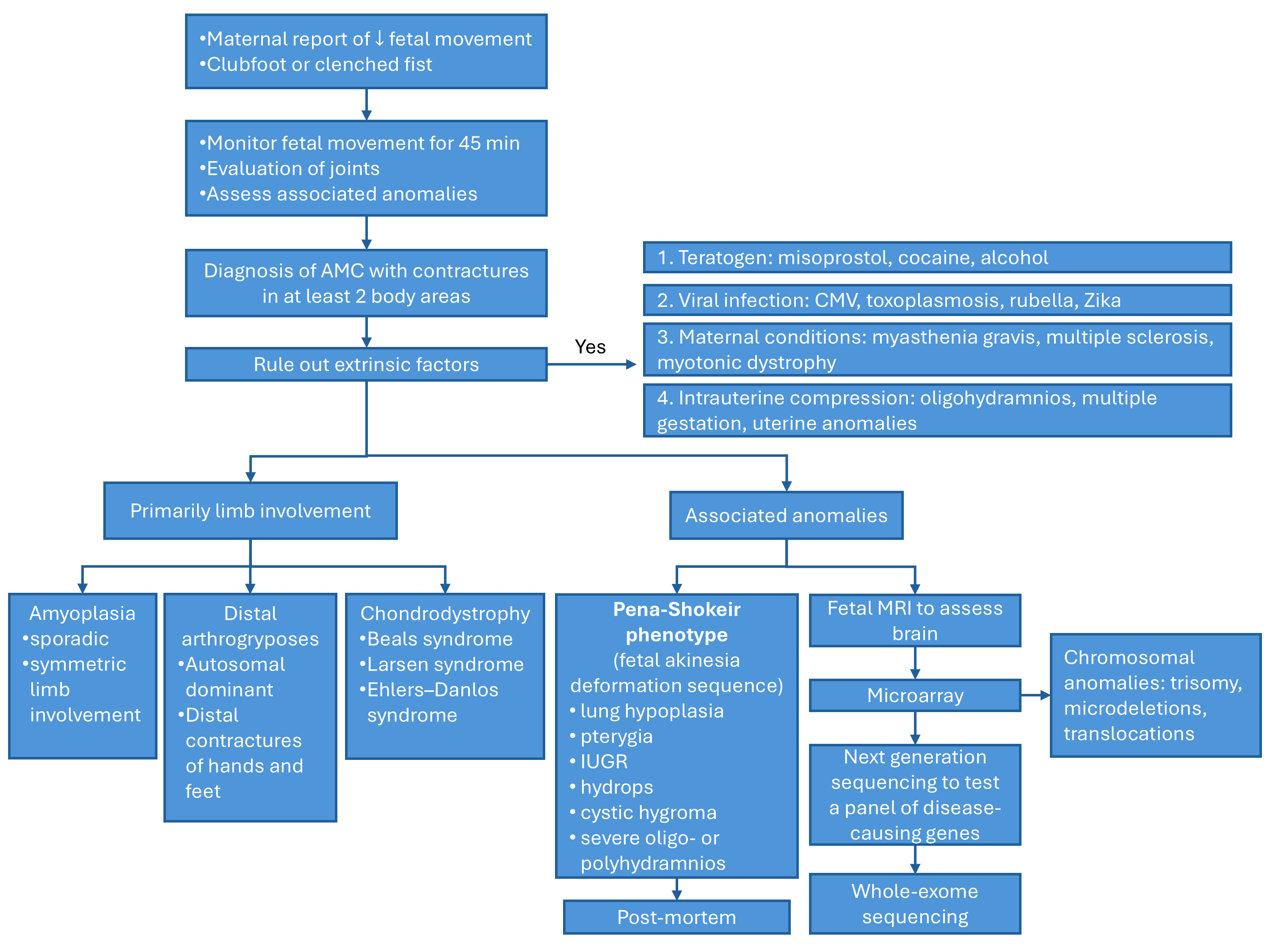
Given the extensive list of conditions that can lead to arthrogryposis multiplex congenita, identifying the underlying disease process in utero can be challenging. Filges et al discuss an approach to diagnose the etiology [13]. Once the diagnosis of arthrogryposis is established by observing decreased fetal movement and joint contractures in two body areas, a careful history and ultrasound examination should assess for any extrinsic fetal conditions that may be contributing to disease (Figure 1). A careful history should be obtained for maternal conditions, teratogen exposures, and viral infections or fever. Ultrasound can evaluate for oligohydramnios, uterine anomalies, and multiple gestation that can cause intrauterine compression. Once extrinsic conditions have been ruled out, ultrasound should be used to determine the body regions involved in contractures, joint positioning, and associated anomalies. If primarily the joints are involved without significant associated anomalies, the differential diagnosis includes amyoplasia, distal arthrogryposes or chondrodystrophies. If the condition affects other organ systems, consider syndromic etiologies. Findings concerning for a lethal condition include lung hypoplasia, pterygia, intrauterine growth restriction, nuchal edema, hydrops, severe oligo- or polyhydramnios, and are collectively referred to as the fetal akinesia deformation sequence or classic Pena-Shokeir phenotype [24]. Central nervous system malformations are also associated with worse prognosis. Fetal MRI may be appropriate to further characterize brain structures and assess for lung hypoplasia [13].
As of 2019, over 400 genes have been identified in arthrogryposis [25] and genetic mutations are found in 53-58% of patients [11,26]. Approximately 40% of mutated genes involve proteins that affect skeletal muscle, while 22% affect the brain, and 17% affect the neuromuscular junction [11]. Given the extensive list of conditions that could cause arthrogryposis multiplex congenita, genetic evaluation is complex. Microarray could be used as a first line test, particularly for individuals with intellectual disability or consanguinity, as chromosomal disorders such as trisomy 18, mosaic trisomy 8, and microdeletions including contiguous gene deletion syndromes could be detected [1,13]. If the workup is negative, consider next generation sequencing for testing a clinically validated panel of disease-causing neuromuscular genes. A genetic diagnosis in a cohort of patients with fetal akinesia, arthrogryposis, and severe congenital myopathies was obtained in 47% of families using this technique [27]. Finally, whole genome sequencing studies can be offered given the expanding number of disease-causing genes in arthrogryposis multiplex congenita [5,11].
In case of fetal demise or termination of pregnancy, postmortem exam could help identify the cause of arthrogryposis and offer the family information on the recurrence risk in subsequent pregnancies [28]. In a study reviewing 132 autopsies, the estimated risk of recurrence changed in almost one-third of families [28]. When known entities have been excluded, an empiric recurrence risk for unaffected parents of an affected child is 3–5%. Central nervous system involvement may increase the risk to 7% [20].
Prognosis is variable depending on the underlying disease process causing arthrogryposis multiplex congenita. Factors that may complicate the neonatal period include osteopenia, jaw or spine involvement, pulmonary hypoplasia, and hypotonia. Osteopenia leads to an increased risk of fracture, which is approximately 9% at delivery. There is no indication for cesarean section as the rate of bone fractures does not differ significantly between vaginal (6%) and cesarean (11%) delivery [6]. Pulmonary hypoplasia, severe kyphosis or scoliosis, and profound hypotonia can result in poor ventilation. Aspiration and difficult intubation may be encountered in patients with jaw or spine involvement [20]. After birth, aggressive intervention with physical and occupational therapy, as well as orthopedic referral is warranted to improve outcomes. In milder cases, contractures may improve with splinting, passive range of motion, and physiotherapy. Surgical treatment includes tendon reconstruction, extensor lengthening, repositioning, and cast fixation. In scoliosis, spinal stabilization procedures may be necessary [29].
References
[1] Skaria P, Dahl A, Ahmed A. Arthrogryposis multiplex congenita in utero: radiologic and pathologic findings. J Matern Fetal Neonatal Med. 2019 Feb;32(3):502-511.
[2] Wahlig B, Poppino K, Jo CH, et al. Arthrogryposis multiplex congenita: a 28-year retrospective study. Dev Med Child Neurol. 2022 Apr;64(4):476-480.
[3] Lowry RB, Sibbald B, Bedard T, et al. Prevalence of multiple congenital contractures including arthrogryposis multiplex congenita in Alberta, Canada, and a strategy for classification and coding. Birth Defects Res A Clin Mol Teratol. 2010 Dec;88(12):1057-61.
[4] Darin N, Kimber E, Kroksmark AK, et al. Multiple congenital contractures: birth prevalence, etiology, and outcome. J Pediatr. 2002 Jan;140(1):61-7.
[5] Illés A, Pikó H, Bartek V, et al. Heterogenic Genetic Background of Distal Arthrogryposis—Review of the Literature and Case Report. Children (Basel). 2024 Jul 16;11(7):861.
[6] Lemin S, Van Bosse HJP, Hutka L, et al. Prenatal diagnosis (or lack thereof) of arthrogryposis multiplex congenita and its impact on the perinatal experience of parents: A retrospective survey. Prenat Diagn. 2024 May;44(5):614-622.
[7] Filges I, Hall JG. Failure to identify antenatal multiple congenital contractures and fetal akinesia--proposal of guidelines to improve diagnosis. Prenat Diagn. 2013 Jan;33(1):61-74.
[8] Andonotopo W, Medic M, Salihagic-Kadic A, et al. The assessment of fetal behavior in early pregnancy: comparison between 2D and 4D sonographic scanning. J Perinat Med. 2005;33(5):406-14.
[9] Donker ME, Eijckelhof BHW, Tan GMB, et al. Serial postural and motor assessment of Fetal Akinesia Deformation Sequence (FADS). Early Hum Dev. 2009 Dec;85(12):785-90.
[10] Hoellen F, Schröer A, Kelling K, et al. Arthrogryposis multiplex congenita and Pena-Shokeir phenotype: challenge of prenatal diagnosis--report of 21 cases, antenatal findings and review. Fetal Diagn Ther. 2011;30(4):289-98.
[11] Laquerriere A, Jaber D, Abiusi E, et al. Phenotypic spectrum and genomics of undiagnosed arthrogryposis multiplex congenita. J Med Genet. 2022 Jun;59(6):559-567.
[12] de Vries JIP, Fong BF. Normal fetal motility: an overview. Ultrasound Obstet Gynecol. 2006 Jun;27(6):701-11.
[13] Filges I, Tercanli S, Hall JG. Fetal arthrogryposis: Challenges and perspectives for prenatal detection and management. Am J Med Genet C Semin Med Genet. 2019 Sep;181(3):327-336.
[14] Busack B, Eric Ott C, Henrich W et al. Prognostic significance of prenatal ultrasound in fetal arthrogryposis multiplex congenital. Arch Gynecol Obstet. 2021 Apr;303(4):943-953.
[15] Gundogan M, Fong K, Keating S, et al. First trimester ultrasound diagnosis of lethal multiple pterygium syndrome. Fetal Diagn Ther. 2006;21(5):466-70.
[16] Nemec SF, Höftberger R, Nemec U, et al. Fetal akinesia and associated abnormalities on prenatal MRI. Prenat Diagn. 2011 May;31(5):484-90.
[17] Dieterich K, Kimber E, Hall JG. Central nervous system involvement in arthrogryposis multiplex congenita: Overview of causes, diagnosis, and care. Am J Med Genet C Semin Med Genet. 2019 Sep;181(3):345-353.
[18] Pugash D, Oh T, Godwin K, et al. Sonographic ‘molar tooth’ sign in the diagnosis of Joubert syndrome. Ultrasound Obstet Gynecol. 2011 Nov;38(5):598-602.
[19] Hall JG, Aldinger KA, Tanaka KI. Amyoplasia revisited. Am J Med Genet A. 2014 Mar;164A(3):700-30.
[20] Rink BD. Arthrogryposis: a review and approach to prenatal diagnosis. Obstet Gynecol Surv. 2011 Jun;66(6):369-77.
[21] Sung SS, Brassington AME, Grannatt K, et al. Mutations in genes encoding fast-twitch contractile proteins cause distal arthrogryposis syndromes. Am J Hum Genet. 2003 Mar;72(3):681-90.
[22] Coelho KE, Sarmento MF, Veiga CM, et al. Misoprostol embryotoxicity: clinical evaluation of fifteen patients with arthrogryposis. Am J Med Genet. 2000 Dec 11;95(4):297-301.
[23] Lavi E, Montone KT, Rorke LB, et al. Fetal akinesia deformation sequence (Pena-Shokeir phenotype) associated with acquired intrauterine brain damage. Neurology. 1991 Sep;41(9):1467-8.
[24] Pena SD, Shokeir MH. Syndrome of camptodactyly, multiple ankyloses, facial anomalies, and pulmonary hypoplasia: a lethal condition. J Pediatr. 1974 Sep;85(3):373-5.
[25] Kiefer J, Hall JG. Gene ontology analysis of arthrogryposis (multiple congenital contractures). Am J Med Genet C Semin Med Genet. 2019 Sep;181(3):310-326.
[26] Bayram Y, Karaca E, Akdemir ZC, et al. Molecular etiology of arthrogryposis in multiple families of mostly Turkish origin. J Clin Invest. 2016 Feb;126(2):762-78.
[27] Todd EJ, Yau KS, Ong R, et al. Next generation sequencing in a large cohort of patients presenting with neuromuscular disease before or at birth. Orphanet J Rare Dis. 2015 Nov 17:10:148.
[28] Boyd PA, Tondi F, Hicks NR, et al. Autopsy after termination of pregnancy for fetal anomaly: retrospective cohort study. BMJ. 2004 Jan 17;328(7432):137.
[29] Van Bosse HJP, Zlotolow DA. The Orthopaedic Management of Arthrogryposis Multiplex Congenita. Journal of JPOSNA. 2021 May;3(2):277.
Discussion Board
Winners

Irina Morozova Russian Federation Physician

Dianna Heidinger United States Sonographer

Javier Cortejoso Spain Physician

Chursina Olga Russian Federation Physician

Andrii Averianov Ukraine Physician

Ana Ferrero Spain Physician

Alexandr Krasnov Ukraine Physician

Mayank Chowdhury India Physician

Vladimir Lemaire United States Physician

Ivan Ivanov Russian Federation Physician

Boujemaa Oueslati Tunisia Physician

Tatiana Koipish Belarus Physician

carlos lopez Venezuela Physician

CHARLES SARGOUNAME India Physician

Miğraci Tosun Turkey Physician

Peter conner Sweden Physician

Kimberly Delaney United States Sonographer

Marianovella Narcisi Italy Physician

CHEN YANG China Physician

Amparo Gimeno Spain Physician

Elena Andreeva Russian Federation Physician

Basem Hamed United States

Muradiye YILDIRIM Turkey Physician

ALBANA CEREKJA Italy Physician

Eti Zetounie Israel Sonographer

Murat Cagan Turkey Physician

Sonio Sonio France AI

ANA PAULA PASSOS Brazil Physician

Büşra Cambaztepe Turkey Physician

Ionut Valcea Romania Physician

Sviatlana Akhramovich Belarus Physician

Halil Korkut Dağlar United States Physician

Hien Nguyen Van Viet Nam Physician

Miguel Sanchez Mexico Physician

Almaz Kinzyabulatov Russian Federation Physician

Kareem Haloub Australia Physician

Zuzana Briešková Slovakia Physician

Ann-Christin Dr. Sönnichsen Germany Physician

Annette Reuss Germany Physician
shruti Agarwal India Physician

CHERYL TURNER United States Sonographer

shay kevorkian Israel Physician

Sruthi Pydi India Physician

Veronika Bartkovjaková Slovakia Physician

Rupal Sasani India Physician

Denys Saitarly Israel Physician

Le Tien Dung Viet Nam Physician

Tetiana Ishchenko Ukraine Physician

Costin Radu Lucian Romania Physician

María Victoria Peral Parrado Spain Physician

Le Duc Viet Nam Physician

Zuzana Rak Slovakia Physician

Hana Habanova Slovakia Physician
Gnanasekar Periyasamy India Physician

Jagdish Suthar India Physician

DR RAJESH KAMBLE India Physician

Hân Đỗ Viet Nam Physician

ASHLEA HARDIN United States Sonographer

Ayse Ceren Duymus Turkey Physician

Manuel Rodriguez Mexico Physician

ZHANNA Kurmangaliyeva Kazakhstan Physician

Murad Esetov Russian Federation Physician

Crystal Eskew United States Sonographer

Mukesh Kannan India Consultant radiologist

Dubyanskaya Yuliya Russian Federation Physician

Surekha Bhimangouda India Physician

Juli Hamilton United States Sonographer

Bogdan Panaitescu United States Physician

Homam Saker United States Physician