Case of the Week #577
Australia
Case Report: A 25-year-old primigravida in a nonconsanguineous relationship presents for routine screening. Her past medical and family history are unremarkable. Initial screening was described as normal. The second screening was delayed until 25-26 weeks of gestation and shows the following findings.
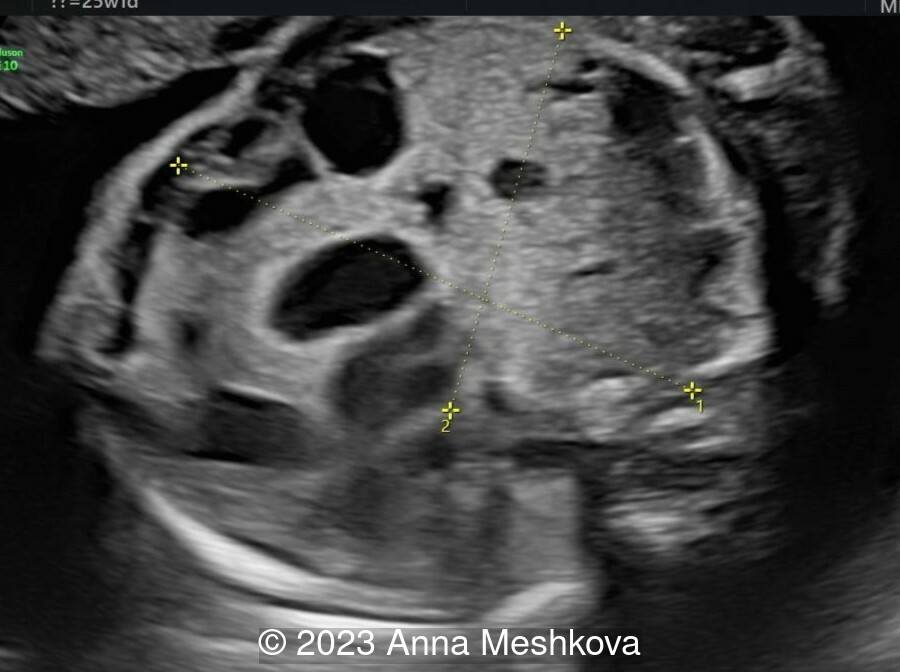
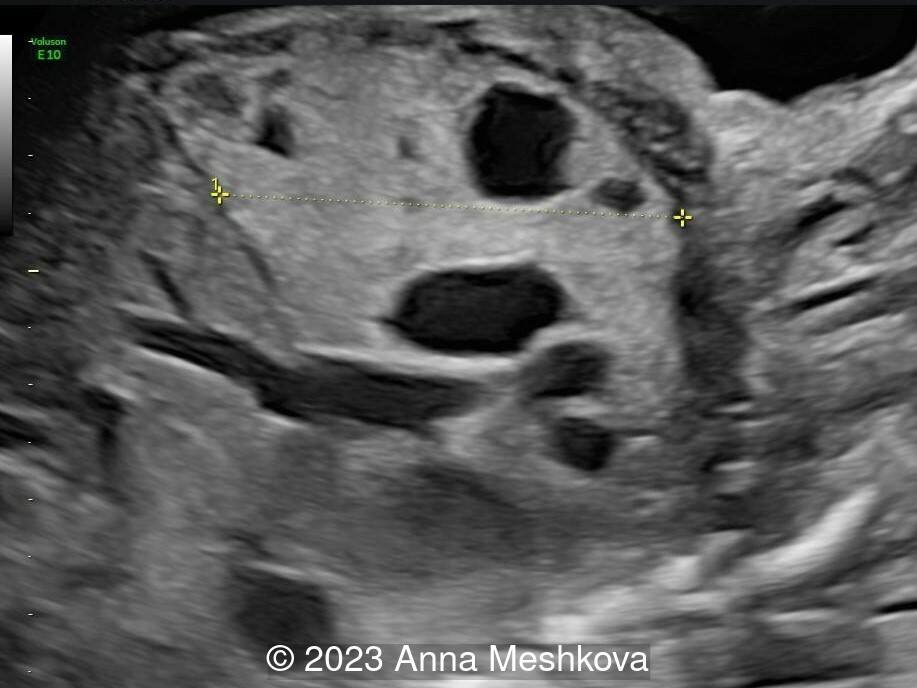
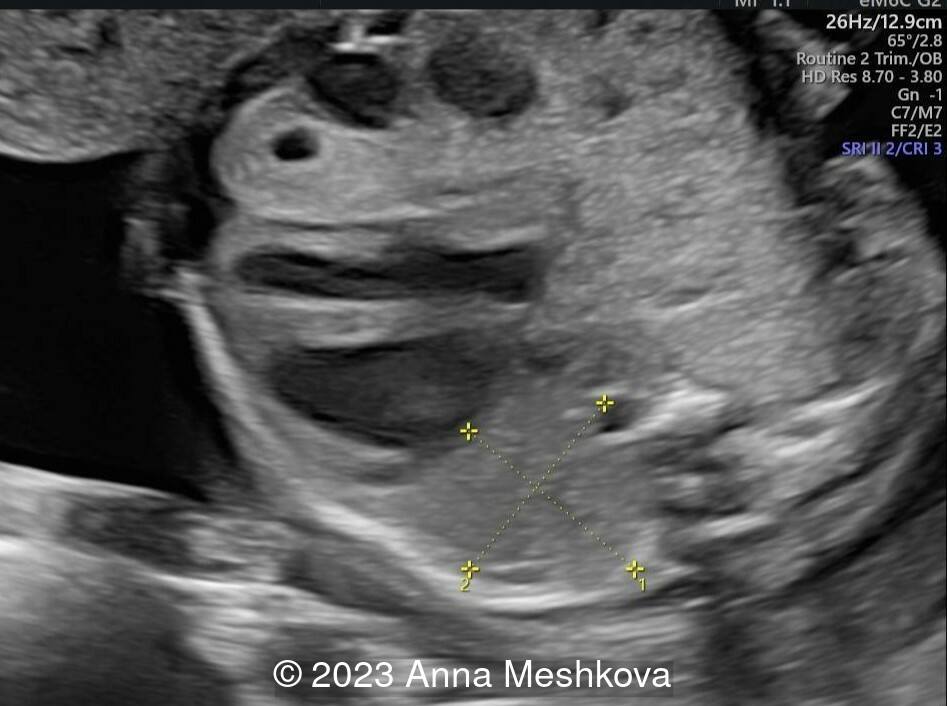
View the Answer Hide the Answer
Answer
We present a case of Congenital cystic adenomatoid malformation (CCAM) of the right lung.
Discussion
Congenital cystic adenomatoid malformations (CCAM) of the lung arise from excessive proliferation of tubular bronchial structures [1]. This condition was first described by Chi’n Tang in 1949 [2]. It is a non-hereditary, hamartomatous abnormality of the lung with unknown etiology. Development of human lung is subdivided into embryonal (3-7 weeks), pseudoglandular (7-17 weeks), canalicular (17-29 weeks), saccular (24-36 weeks), and alveolar (36 weeks to maturity). CCAM develops during the pseudoglandular and saccular periods (7-35 weeks) [1]. CCAM has an incidence of 1 in 25,000 to 35,000 pregnancies and represents 25% of congenital lung malformations [3]. Males and females are equally affected. Eighty percent of the lesions are recognized in the neonatal period, however, there are reports in the adult population [4]. CCAM lesions are usually unilateral and restricted to a single lobe [5]. In up to 10% of cases, additional extra-pulmonary abnormalities can be found, such as renal, central nervous system, gastrointestinal, and cardiac defects [6].
In 1977, CCAM was classified into three subtypes by Stocker et al [7]. Type I lesions constitute 50-70% of cases and are single or multiple large cysts (>2cm) lined by flattened, cuboidal cells frequently producing mediastinal herniation. The walls of the cysts contain prominent smooth muscle and elastic tissue. Mucus-producing cells can be seen, though the presence of cartilage is extremely rare. Mucin production is unique to type I lesions. Type II lesions constitute 15-30% and are multiple small cysts (<2cm), lined by ciliated cuboidal to columnar epithelium with a structure resembling that of respiratory bronchioles. Distended alveoli are present between the epithelium-lined cysts. Mucus cells and cartilage are not seen. This type is usually associated with other systemic anomalies including renal anomalies (bilateral renal agenesis, abnormalities of the ureter, bladder, and urethra), abdominal wall defects, diaphragmatic hernia, central nervous system defects (hydrocephalus), spinal deformities, gastrointestinal defects (jejunal atresia, tracheoesophageal fistula, and imperforate anus), cardiac anomalies (ventriculoseptal defects, tetralogy of Fallot, and truncus arteriosus), syringomyelia, and anomalies of reproductive tract. Type III lesions constitute 5-10% and are usually large, bulky, non-cystic lesions producing mediastinal shift. Bronchial-like structures are lined by ciliated cuboidal epithelium and separated by masses of alveolar-sized structures lined by non-ciliated cuboidal epithelium [8]. In 2002, Stocker expanded CCAM into 5 subtypes and renamed the condition to congenital pulmonary airway malformation (CPAM) [9].
On ultrasound, CCAM presents as a multicystic lesion within the lung field. Differential diagnosis includes diaphragmatic hernia, bronchogenic cyst, cystic fibrosis, congenital lobar emphysema, and pulmonary sequestration. CCAM communicates with the tracheobronchial tree and derives its blood supply from pulmonary circulation. In contrast, pulmonary sequestration contains immature lung tissue without connection to tracheobronchial tree and derives its blood supply from an aberrant systemic blood vessel.
For lung masses, prognosis is determined by the fetal lung mass size (a single measurement of lung mass at the maximum diameter) or by the CCAM volume ratio (CVR). CCAM volume is calculated by using a formula for prolate ellipse, with measurements of lung mass in three perpendicular planes. The CVR is calculated by dividing the CCAM volume by head circumference measured in centimeters. Thus CVR = (length × depth × width × 0.52 / head circumference) [10]. The size of the lung mass or CVR correlates strongly with the fetal outcome at threshold values of 5.2cm for mass size and 2 for CVR [10]. There was 100% survival of fetuses with a mass value <5.2 cm and CVR <2. A lung mass size of >5.2cm or CVR >2 was associated with marked mediastinal deviation, hydrops, ascites, and heart failure. Fetal echocardiography can be helpful in managing these fetuses. If there are no signs of heart failure, the fetuses may benefit from maternal steroid therapy and serial thoracocentesis. Those fetuses with impending cardiac failure may benefit from fetal surgery [10].
Prognosis also depends on Stocker type. Type I lesions carry an overall good prognosis. In type II lesions, the associated anomalies determine the overall prognosis. Type III lesions carry a poor prognosis as they are usually large and present with cardiovascular compromise. Other factors that contribute to a poor prognosis are bilateral involvement, hydrops and associated congenital anomalies [11]. In a series reviewing 24 fetuses with CCAM, 21 were born alive (there were 2 termination of pregnancy and 1 in utero death), 15 underwent surgical resection, with 1 perioperative death. Overall survival was approximately 80%. Despite in utero regression of the CCAM lesion in 9 fetuses, one developed serious infectious complications after birth [12].
The most common presentation of CCAM in neonates is progressive respiratory distress including tachypnea, grunting, retraction, and cyanosis. Complications include compression of the mediastinal structures producing cardiovascular compromise. Serial antenatal sonographic evaluation, good obstetric care, and delivery at a tertiary care center are the preferred plan of treatment for antenatally detected cases. In adults, CCAM usually presents as recurrent chest infections such as pneumonia, abscess formation, and fungal infections. Additional complications include spontaneous pneumothorax, hemoptysis, air embolism, intralobar sequestration, and development of bronchogenic carcinoma. Chest X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) are helpful in the diagnosis. Lobectomy is the treatment of choice for symptomatic cases.
References
1. Sood M, Sharma S. Congenital cystic adenomatoid malformation of lung-A case report. Currt Pediatr Res. 2011;15:61–3.
2. Khan NU, Jones MT, Greaves M. Case report: Congenital cystic adenomatoid malformation of an entire lung in a 33-year-old man: A case report and review of the literature. Br J Radiol. 2008 Nov;81(971):e276-8.
3. Laberge JM, Flageole H, Pugash D, et al. Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: a Canadian experience. Fetal Diagn Ther. 2001 May-Jun;16(3):178-86.
4. Feng A, Cai H, Sun Q, et al. Congenital cystic adenomatoid malformation of lung in adults: 2 rare cases report and review of the literature. Diagn Pathol. 2012 Apr 3;7:37.
5. Davenport M, Warne SA, Cacciaguerra S, et al. Current outcome of antenally diagnosed cystic lung disease. J Pediatr Surg. 2004 Apr;39(4):549-56.
6. Stocker JT. Congenital and developmental diseases. In: Dail DH, Hammer SP, editors. Pulmonary pathology, New York: Springer. 1994; 2nd ed:174-180.
7. Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol. 1977 Mar;8(2):155-71.
8. Sfakianaki AK, Copel JA. Congenital cystic lesions of the lung: Congenital cystic adenomatoid malformation and bronchopulmonary sequestration. Rev Obstet Gynecol. 2012;5(2):85-93.
9. Stocker JT. Congenital pulmonary airway malformation: A new name and an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathol. 2002;41:424–31.
10. Cass DL, Olutoye OO, Cassady CI, et al. Prenatal diagnosis and outcome of fetal lung masses. J Pediatr Surg. 2011 Feb;46(2):292-8.
11. Annam V, Korishetty SI, Yelikar BR, et al. Bilateral congenital cystic adenomatoid malformation, stocker type III with associated findings and review of literature. Indian J Pathol Microbiol. 2010 Apr-Jun;53(2):331-3.
12. Chow PC, Lee SL, Tang MHY, et al. Management and outcome of antenatally diagnosed congenital cystic adenomatoid malformation of the lung. Hong Kong Med J. 2007 Feb;13(1):31-9.
Discussion Board
Winners

Dianna Heidinger United States Sonographer

Javier Cortejoso Spain Physician

Danilo Feitosa Brazil Physician

Umber Agarwal United Kingdom Maternal Fetal Medicine

Igor Yarchuk United States Sonographer

Iuliia Iudina Russian Federation Physician

Larysa Gazarova United States Physician

Andrii Averianov Ukraine Physician

Ana Ferrero Spain Physician

Alexandr Krasnov Ukraine Physician

Adrian Popa Romania Physician

filiz halici öztürk Turkey Physician

Oskar Sylwestrzak Poland Physician

Vladimir Lemaire United States Physician

Shilpen Gondalia India Physician

Ivan Ivanov Russian Federation Physician

Boujemaa Oueslati Tunisia Physician

CHARLES SARGOUNAME India Physician

Aysegul Ozel Turkey Physician

Halil Mesut Turkey Physician

Rushina Patel United States Sonographer

Olivia Ionescu United Kingdom Physician

Rebecca Evans Australia Sonographer

Suat İnce Turkey Physician

Shari Morgan United States Sonographer

Liem Dang Le Viet Nam Physician

Amparo Gimeno Spain Physician

Elena Andreeva Russian Federation Physician

Patrícia Silva Portugal Physician

Muradiye YILDIRIM Turkey Physician

Dimitrios Spiliopoulos Greece Physician

doğa öcal Turkey Physician

ALBANA CEREKJA Italy Physician
Selvanandhini Gopalasundaram India Physician

Murat Cagan Turkey Physician

Mária Brešťanská Slovakia Physician

Büşra Cambaztepe Turkey Physician
Rasha Abo Almagd Egypt Physician

Inass Osman United Kingdom Physician
Gayane Begjanyan Armenia Physician

Ionut Valcea Romania Physician

Ragıp Atakan Al United States Physician

Sviatlana Akhramovich Belarus Physician

Prakriti Patil India Physician
KHALED RAMADAN Egypt Physician

Abdullah Alnoman Canada Physician

Borisova Elena Russian Federation Physician

Miguel Sanchez Mexico Physician

CHANDRAKANTH K S India Physician
Martina Vagaská Slovakia Physician

Hannah Danielson United States Sonographer

Dr.Mayuri Dixit India Physician

Mariam Bamanie Saudi Arabia Physician

Maria Chiara Marra Italy Physician

Vandana Yakub India Physician

Arati Appinabhavi India Physician

BHOOMIKA SHARMA India Physician

Janoub Khazaal France Physician

Vu The Anh Viet Nam Physician

CHERYL TURNER United States Sonographer
Ebtihal Bin jomaa Libyan Arab Jamahiriya Physician

Mahvish Suria Australia Sonographer

Saradindu Mandal India Physician

Perrine Riou-Kerangal French Polynesia Sage-femme échographiste

Yihui Chen Singapore Sonographer

Erdem Fadiloglu Turkey Physician
Narmada Damodaran India Physician

Deniz OLUKLU Turkey Physician

Mai Phương Viet Nam Physician

Yogesh Chinnawar India Physician

Katherine Gorman United States Sonographer

Paula Abubakar Ghana Sonographer

Dr.Kuldeep Raykhere India Physician